Manager : Sébastien CARNICELLA
Keywords
Themes of research
The basal ganglia are a collection of interconnected subcortical nuclei critical for reward processing and the generation of goal-directed-behaviors. They include structures as the striatum, pallidum, or the subthalamic nucleus), and are under the influence of several neuromodulators with a pivotal role of dopamine. A plethora of neuropsychiatric symptoms in brain disorders are underlain by dysfunctions within these circuits. This encompasses maladaptive and compulsive seeking in drug addiction, motivational fluctuation in PD, or abnormal hedonic processes in drug withdrawal, depression, pain or feeding disorders, and many more. Deep Brain Stimulation (DBS) hold promise to develop new focused therapies, but the neural circuits and neurobiological mechanisms underlying these symptoms and the effect of DBS remain unclear. Moreover, neuropsychiatric symptoms can precede the diagnostic of neurodegenerative diseases, as for apathy or depression in patients with Parkinson’s Disease. The common mechanisms between neuropsychiatric and neurodegenerative disease represent a hot topic in current neurosciences, but too few questions have been answered so far.
Thus, not only does elucidating the underlying mechanisms of neuropsychiatric symptoms and in particular motivation impairment will contribute to develop new targeted therapies to improve the quality of life of patients, but it will also permit to identify predictor biomarkers allowing early preventive treatments or disease modifying approaches.
Our translational approaches, from the molecules to the patients, is organized in 4 interconnected axes:
1. Modulating reward circuit function with deep brain stimulation to treat neuropsychiatric diseases (PI: Dr. Yvan Vachez).
2. Metabolomic and neuropsychiatric markers for PD early diagnosis and pathophysiology (PI: Pr. Sabrina Boulet).
3. Investigation of striatal circuits adaptations underlying addiction-related behaviors: transcriptomic markers and mechanistic approaches (PI: Dr. Sebastien Carnicella).
4. Investigating the mechanistic of pharmacotherapies for alcohol use disorders and dimensional approaches of addiction for translational research (PI: Pr. Maurice Dematteis).
Our Approaches
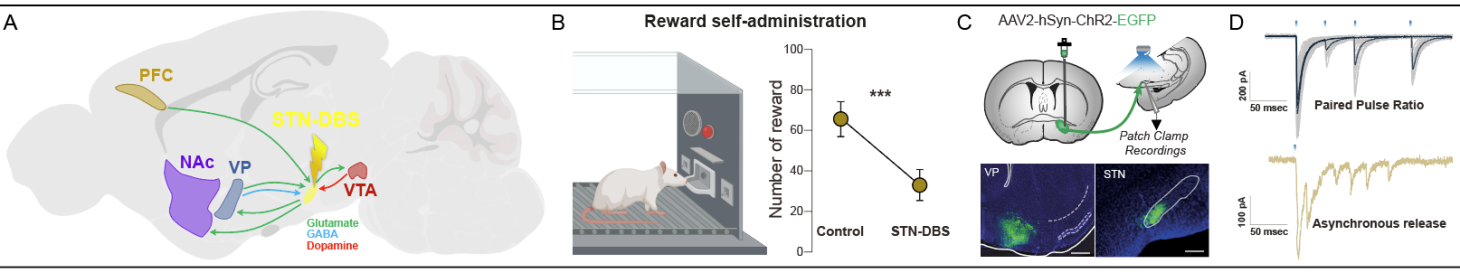
1. Modulating reward circuit function with deep brain stimulation to treat neuropsychiatric disease (PI: Dr. Yvan Vachez). DBS is one of the major breakthroughs over the last 40 years in terms of treatments for movement disorders, and it holds promise for the development of new circuit-focused therapies for neuropsychiatric disorders. However, the behavioral, circuit and synaptic effects of DBS remain to be fully elucidated. The objective is to dissect and disentangle the neuronal substrates and subcircuits responsible respectively for the deleterious and beneficial impacts of DBS in different rodent models of pathological motivated behaviors (i.e., apathy-like in PD, addictions…etc…). We combine experimental DBS, retrograde viral vectors strategies, neuromodulatory technics as chemogenetic, ex vivo patch clamp electrophysiology coupled with optogenetic and in vivo calcium photometry to characterize the synaptic adaptations in specific reward circuits in these pathological and DBS contexts. The overarching goal is to leverage insight from these circuit studies to optimize DBS for PD and to provide blueprints for novel targeted therapies for neuropsychiatric disorders.
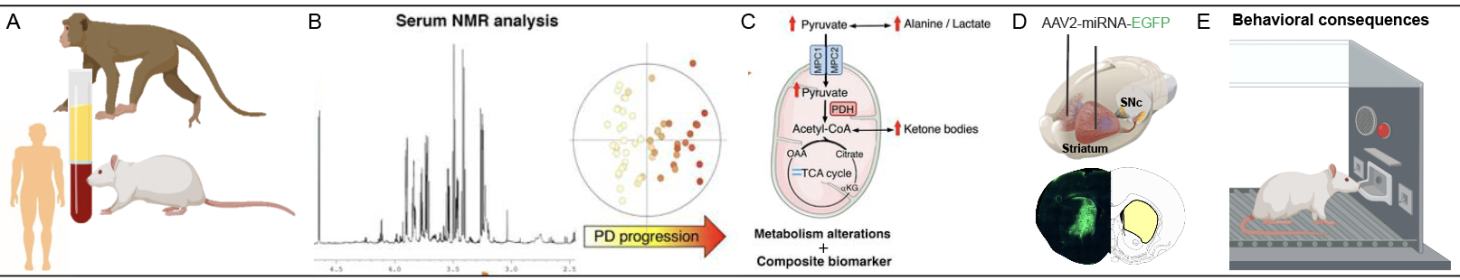
2. Metabolomic and neuropsychiatric markers for PD early diagnosis and pathophysiology (PI: Pr. Sabrina Boulet). Beyond the cardinal motor features of the disease, PD is associated with several behavioral complications. Apathy, or loss of motivation, is a symptom frequently observed in early stages of PD, including in undiagnosed patients who will develop the disease. We recently identified a blood biomarker predicting the early diagnosis of PD and the goal of this axis is to investigate the role of this early metabolism dysfunction for the progression of PD and the prodromal motivational disorders. We use biological samples from multiple animal models of PD along samples from de novo human patients, and combine metabolomic approaches, conditional knock-down and animal behavior through collaboration with Dr. Florence Fauvelle (Lemasson/Christen team) and with a well-established network of clinicians. We aim at leveraging the characterization of this metabolism alteration to 1) propose new therapeutic avenues for the premotor neuropsychiatric symptoms of PD and 2) to emulate disease modifying therapies to slow-down or stop the progression of PD.
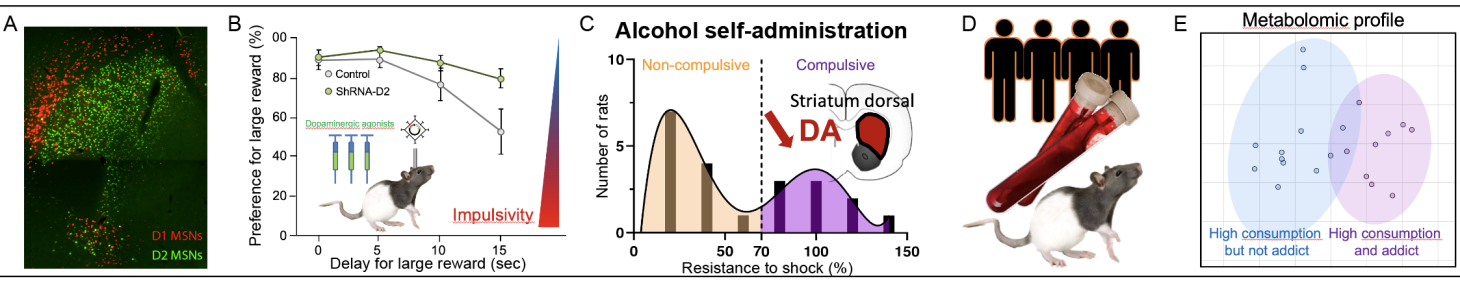
3. Investigation of striatal circuits adaptations underlying addiction-related behaviors: transcriptomic markers and mechanistic approaches (PI: Dr. Sebastien Carnicella). Compulsivity, the uncontrolled continuation of a behavior such as drug seeking despite the negative consequences is the hallmark of drug or behavioral addiction. The striatum is strongly implicated for these abnormal motivated behaviors but circuits and mechanisms are still not clear. The aim is to identify the synaptic, cellular and intracellular dysfunctions in the striatal neuronal populations, in particular through DA signaling alterations, and their role in the development of impulsive and compulsive behaviors related to alcohol addiction or behavioral addiction in PD. We use chemogenetic and conditional approaches in rats, cellular and molecular imaging, multiple behavioral paradigms, and ex vivo patch clamp electrophysiology to dissect the corticostriatal adaptations underlying these pathological motivated behaviors. We complete these approaches with blood samples analysis from patients with drug addiction or with PD and behavioral addiction, to validate the mechanistic characterizations and to identify predictive biomarkers.
4. Investigating the mechanistic of pharmacotherapies for alcohol use disorders and dimensional approaches of addiction for translational research (PI: Pr. Maurice Dematteis). Some breakthroughs have been recently made in the pharmacotherapeutic strategies for alcohol use disorders (AUDs) and addiction, such as the use of baclofen and other repurposed drugs. However, the underlying mechanisms of the beneficial actions of these molecules in AUDs remain largely unknown and also depend on the profile (endophenotype) of the patient. The main objective is therefore to develop and operationalize a systematic dimensional approach in the patient and animal model of AUDs to have a full and transposable behavioral characterization of individuals in clinical and preclinical research. It will build a solid basis to move toward a clinic to the bench and back to clinic strategy for investigating in the lab the therapeutic mechanisms of innovative molecules for AUDs in particular and addiction in general, as well as for the application of an efficient individualized objectively targeted medical approach.
Technics
Behavioral assays: evaluation of mood-related, Pavlovian, operant and motor behaviors in animal models of neuropsychiatric and neurodegenerative disorders.
Conditional viral approaches: AAV stereotactic injection inducing the expression of transgene in anatomically, genetically and molecularly defined cell population.
Neuromodulation: brain structure or circuit neuromodulation with chemogenetic or intracranial drug delivery.
Experimental deep brain stimulation: implantable microstimulators for chronic and long-term stimulation in freely-moving rats.
Ex vivo electrophysiology: neuronal and synaptic activity and plasticity monitoring with patch clamp recordings coupled with optogenetic and pharmacology
Histology and neuroanatomy: viral-genetic tracing, immunohistochemistry, immunofluorescence, optical microscopy.
Biochemistry: protein, RNA or neurotransmitters quantification with western blot, RT-qPCR, Elisa tests…etc…
Additional technics in collaboration: Blood and tissue metabolomics analysis (collab Florence Fauvelle, Lemasson/christen team), in vivo brain electrophysiology (collab Véronique Coizet, Bastin team).
Collaborations
Grenoble Institute of Neurosciences
Dr. Florence Fauvelle
Dr. Véronique Coizet
Dr. Homaira Nawabi
Dr. Stéphane Belin
Dr. Clément Hébert
University of Picardie, Groupe de recherche sur l’alcool et les pharmacodépendances
Pr. Mickael Naasila
Pr. Olivier Pierrefiche
Dr. Sami Ben Hamida
Dr. Jérôme Jeanblanc
University of Poitiers, Laboratoire de Neurosciences Expérimentales et Cliniques
Pr. Pierre Olivier Fernagut
University of Marseille, Institut de Neurobiologie de la Méditerranée
Dr. Olivier Manzoni
University of Montpellier, Institut de Génomique Fonctionnelle
Dr. Emmanuel Valjent
University of Cambridge, Department of Psychology
Pr. David Belin
University of Paris, Institut du Cerveau et de la Moëlle Épinière
Pr. Jean Christophe Corvol
University of Limoges, Centre de Compétence Neurogénétique
Pr. Jean-Luc Houeto
University of Rome, Foundation Santa Lucia
Dr. Paola Bossu
Western Michigan University, School of Medicine
Pr. Jerry Colca
Members
- Principal Investigators
- Pr. Sabrina BOULET, PhD (PU)
- Dr. Sebastien CARNICELLA, PhD (DR)
- Pr. Maurice DEMATTEIS, MD, PhD (PUPH)
- Dr. Colin DERANSART, PhD (MCU-PH)
- Dr. Lucie PENNEL, MD (PH)
- Dr. Yvan VACHEZ, PhD (CR)
- Post Doc
- Dr. Fanny JOLY, PhD
- Dr. Yury VELHO MARTINS LAGES, PhD
- Dr. Lucinda SPEERS, PhD
- PhD Candidates
- Vanille MILLASSEAU (3rd year)
- Mylène WILT (2nd year)
- Cécile Gaudart (1st year)
- Graduate students
- Sepehr Bahrinejad (2nd year)
- Luca Fernandez de Jesu (1st Year)
- Undergraduate students
- Administrator
- Margaret Pras
- Alumni
Publications
Pramipexole restores behavioral inhibition in highly impulsive rats through a paradoxical modulation of frontostriatal networks . Magnard R, Fouyssac M, Vachez YM, Cheng Y, Dufourd T, Carcenac C, Boulet S , Janak PH, Savasta M, Belin D and Carnicella S (2024) Translational Psychiatry.
Hypodopaminergic state of the nigrostriatal pathway drives compulsive alcohol use . Goutaudier R, Joly F, Mallet D, Bartolomucci M, Guicherd D, Carcenac C , Vossier F, Dufourd T, Boulet S,Deransart C, Chovelon B, Carnicella S (2023). Molecular Psychiatry. Jan;28(1):463-474.
Re-routing Metabolism by the Mitochondrial Pyruvate Carrier Inhibitor MSDC-0160 Attenuates Neurodegeneration in a Rat Model of Parkinson's Disease. Mallet D, Goutaudier R ,Barbier EL, Carnicella S, Colca JR, Fauvelle F, Boulet S (2022) Molecular Neurobiology Oct;59(10):6170-6182
Dopamine D3 Receptors: A Potential Target to Treat Motivational Deficits in Parkinson's Disease . Favier M, Carcenac C, Savasta M, Carnicella S (2022) Current Topics in Behavioral Neurosciences. 60:109-132
A metabolic biomarker predicts Parkinson's disease at the early stages in patients and animal models. Mallet D, Dufourd T, Decourt M, Carcenac C, Bossù P,Verlin L, Fernagut PO, Benoit-Marand M, Spalletta G, Barbier EL,Carnicella S , Sgambato V , Fauvelle F , Boulet S (2022) Journal of Clinical Investigation 15;132(4):e146400
Ventral arkypallidal neurons modulate firing to promote reward consumption . Vachez YM, Tooley J, Abiraman K, Matikainen-Ankney B, Casey E, Earnest T, Silberberg H, Godynyuk E, Uddin O, Marconi L, Le Pichon C and Creed MC. (2021). Nature Neuroscience. Mar;24(3):379-390.
Compound 21, a two-edged sword with both DREADD-selective and off-target outcomes in rats. Goutaudier R, Coizet V, Carcenac C, Carnicella S (2020) Plos One Sep 18;15(9):e0238156.
Subthalamic Nucleus Stimulation Impairs Motivation: Implication for Apathy in Parkinson's Disease . Vachez Y, Carcenac C, Magnard R, Kerkerian-Le Goff L, Salin P, Savasta M, Carnicella S, Boulet S (2020) Mov Disord Apr;35(4):616-628.
GPCR and Alcohol-Related Behaviors in Genetically Modified Mice. Neasta J, Darcq E, Jeanblanc J, Carnicella S, Ben Hamida S. (2020) Neurotherapeutics. Jan;17(1):17-42
DREADDs: The Power of the Lock, the Weakness of the Key. Favoring the Pursuit of Specific Conditions Rather than Specific Ligands . Goutaudier R, Coizet V, Carcenac C, Carnicella S. (2019) eNeuro. Oct 14;6(5):ENEURO.0171-19.2019
Nigrostriatal Dopaminergic Denervation Does Not Promote Impulsive Choice in the Rat: Implication for Impulse Control Disorders in Parkinson's Disease . Magnard R, Vachez Y, Carcenac C, Boulet S, Houeto JL, Savasta M, Belin D, Carnicella S. (2018) Front Behav Neurosci. Dec 13;12:312.
Reversing dopaminergic sensitization . Castrioto A, Carnicella S, Fraix V, Chabardes S, Moro E, Krack P. (2017) Mov Disord. Dec;32(12):1679-1683
Motivation and apathy in Parkinson's disease: implication of dopaminergic D3 receptors . Favier M, Carcenac C, Savasta M, Carnicella S. (2017) Med Sci (Paris). Oct;33(10):822-824.
Psychostimulant effect of dopaminergic treatment and addictions in Parkinson's disease . Delpont B, Lhommée E, Klinger H, Schmitt E, Bichon A, Fraix V, Castrioto A, Quesada JL, Pélissier P, Kistner A, Carnicella S, Lüscher C, Broussolle E, Pollak P, Thobois S, Krack P (2017). Mov Disord. Nov;32(11):1566-1573
Implication of dorsostriatal D3 receptors in motivational processes: a potential target for neuropsychiatric symptoms in Parkinson's disease . Favier M, Carcenac C, Drui G, Vachez Y, Boulet S, Savasta M, Carnicella S. (2017) Sci Rep. Jan 30;7:41589
Trait Impulsivity and Anhedonia: Two Gateways for the Development of Impulse Control Disorders in Parkinson's Disease? . Houeto JL, Magnard R, Dalley JW, Belin D, Carnicella S. (2016) Front Psychiatry May 30;7:91
Emotional manifestations of PD: Neurobiological basis . Castrioto A, Thobois S, Carnicella S, Maillet A, Krack P (2016) Mov Disord Aug;31(8):1103-13
Apathy and Impulse Control Disorders: Yin & Yang of Dopamine Dependent Behaviors . Sierra M, Carnicella S, Strafella AP, Bichon A, Lhommée E, Castrioto A, Chabardes S, Thobois S, Krack P. (2015) J Parkinsons Dis 5(3):625-36
Subthalamic deep brain stimulation differently alters striatal dopaminergic receptor levels in rats . Carcenac C, Favier M, Vachez Y, Lacombe E, Carnicella S, Savasta M, Boulet S (2015) Mov Disord. Nov;30(13):1739-49
Thesis of the team (in french)