- Share
- Share on Facebook
- Share on X
- Share on LinkedIn
Communiqué / Parkinson, Team A.Buisson
On November 19, 2025
A major discovery made at GIN, which opens up new avenues for understanding the long-term side effects of this essential treatment.
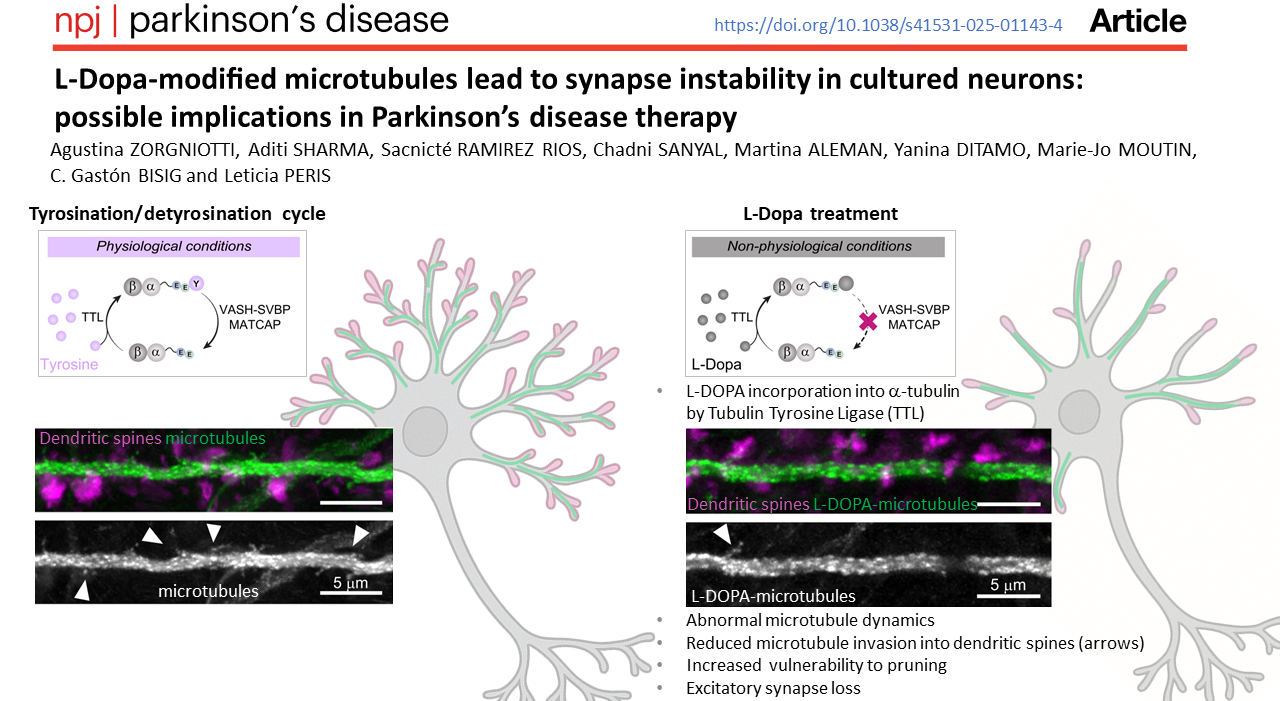
Researchers at the Grenoble Institute of Neurosciences have just revealed a completely unexpected mechanism linked to the standard treatment for Parkinson's disease: L-Dopa. Their study, published in npj Parkinson's Disease, shows that L-Dopa can integrate directly into the microtubules of neurons, disrupting their dynamics and weakening synapses.
For more than 50 years, L-Dopa has remained the most effective treatment for relieving the motor symptoms of Parkinson's disease. However, in many patients, prolonged use leads to symptomatic fluctuations, dyskinesias (involuntary movements), and behavioral disorders.
To understand the mechanisms behind these side effects, Leticia Peris, a neuroscientist at GIN, turned her attention to a little-explored path: the direct impact of L-Dopa on the neuronal cytoskeleton.
What if Parkinson's disease treatment reshaped the synaptic landscape?
Microtubules play a fundamental role in neurons: they support cell structure, ensure intracellular transport and contribute to synaptic plasticity.
The α-tubulin molecule, the building block of microtubules, has a terminal amino acid at its end: tyrosine, which can be removed by VASH-SVBP enzymes or added by the TTL enzyme. Microtubules are therefore regulated by a precise biochemical cycle: the tyrosination/detyrosination cycle, which determines their dynamics. For synapses to function properly, there must be a balance between tyrosinated and detyrosinated α-tubulin.
“This cycle was discovered by Argentine researchers who were my professors when I was a student,” says Leticia Peris. "This study is a continuation of their work. It was carried out in Grenoble, but thanks to a collaboration with my Argentine colleague Gastòn Bisig and Agustina Zorgniotti, who began working on it at the end of her doctoral thesis and continued as a postdoc at GIN. "
Leticia Peris and her team have shown that L-Dopa can be directly incorporated into the end of α-tubulin by the enzyme TTL, in place of tyrosine. This modification has profound consequences because once incorporated, L-Dopa is difficult to remove by VASH-SVBP enzymes. The tyrosination/detylosination cycle is therefore blocked by this new abnormal form of microtubule.
“We have demonstrated that it is indeed the integrated L-Dopa that prevents VASH-SVBP from working properly. This explains why L-Dopa accumulates in microtubules,” explains Leticia Peris.
Researchers also show that microtubules containing L-Dopa become more unstable and less able to enter dendritic spines.
However, this sporadic invasion of dendritic spines by microtubules is essential for their maintenance and for the stability of excitatory synapses. As a result, dendritic spines that are not “visited” by microtubules are eliminated more quickly and the density of excitatory synapses decreases.
A lead for future treatments?
By showing that L-Dopa modifies the tyrosination cycle of microtubules and disrupts the action of VASH-SVBP enzymes, the researchers have identified not only a new mechanism of synapto-toxicity but also a new biological target, and potentially a way to reduce the side effects of long-term treatment.
These results now require in vivo studies to confirm the impact of L-Dopa on the brain's neural circuits. “Looking at the cytoskeleton in neurodegenerative diseases is a new perspective and opens up new therapeutic targets,” concludes Leticia Peris.
Reference :
L-Dopa-modified microtubules lead to synapse instability in cultured neurons: possible implications in Parkinson’s disease therapy
Agustina Zorgniotti, Aditi Sharma, Sacnicte Ramiyrez-Rios, Chadni Sanyal, Martina Aleman, Yanina Ditamo, Marie-Jo Moutin, C. Gastón Bisig, Leticia Peris
npj Parkinsons Dis. 11, 298 (2025). doi.org/10.1038/s41531-025-01143-4
Date
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn