- Share
- Share on Facebook
- Share on X
- Share on LinkedIn
Communiqué / Team I.Arnal/A.Andrieux
On May 27, 2024
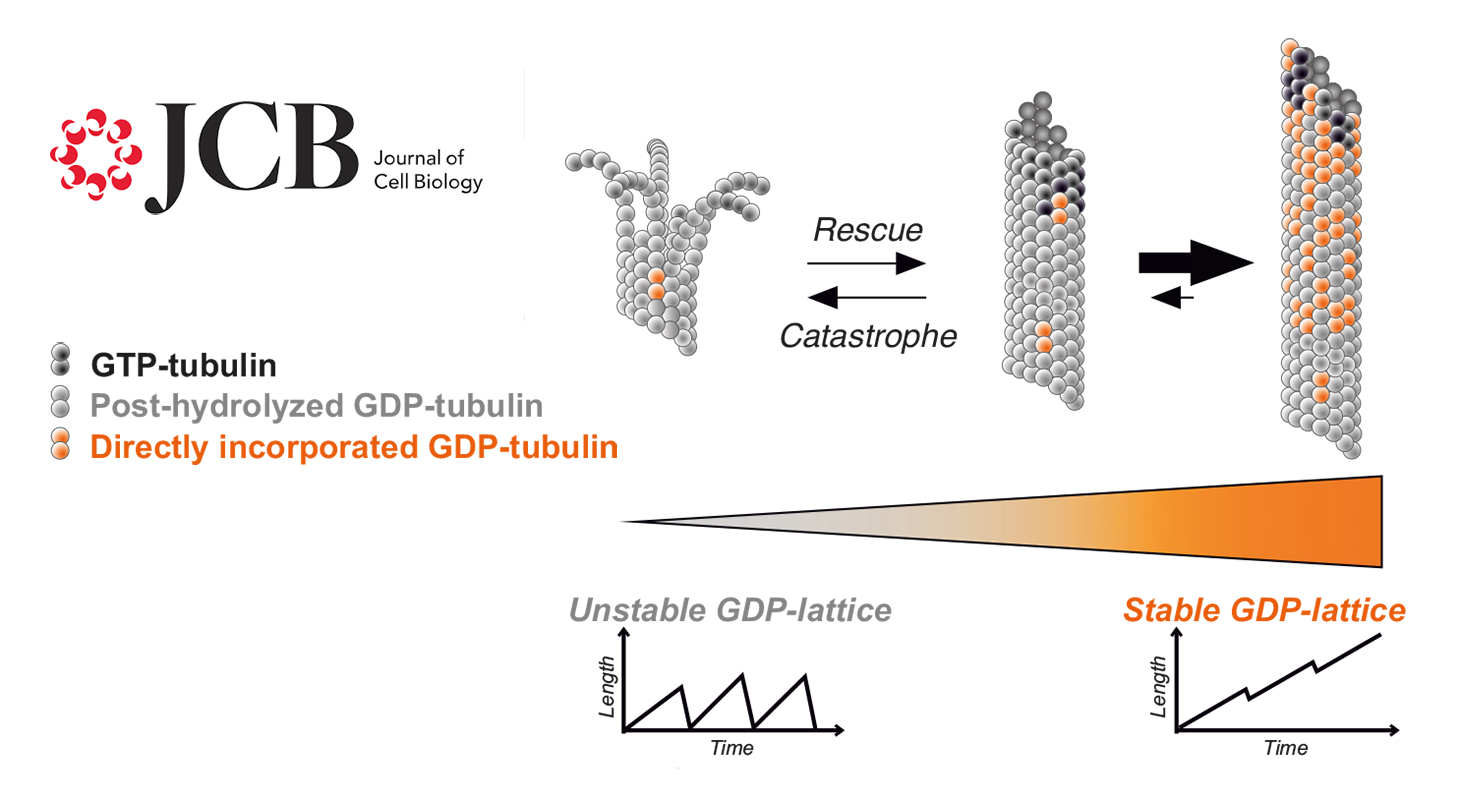
Researchers at the GIN provide a new understanding of the dynamic instability of microtubules.
GDP-bound tubulin, previously considered inactive in microtubule assembly, can actually polymerize into remarkably stable microtubules. These findings have just been published in the Journal of Cell Biology.
The interior of cells contains a network of fibers forming a veritable skeletal structure, called the cytoskeleton. This cytoskeleton plays a crucial role in essential functions such as intracellular transport, maintenance of cell shape, and cell division.
Unlike the bone skeleton, the cytoskeleton is dynamic, meaning it can quickly reorganize in response to the cell's needs. This situation creates a contradictory demand: the cytoskeleton must be stable enough to fulfill its functions while being flexible enough to reorganize into new networks when necessary. Thus, controlling the dynamics of the cytoskeleton is essential for proper cell function.
Microtubules are a major component of the cytoskeleton. They are hollow tubes with a diameter of 25 nm and lengths of a few micrometers, forming "rails" necessary for intracellular transport. Microtubules result from the assembly of a dimeric protein, tubulin, which incorporates at the ends of the tube. The assembly and disassembly of microtubules are governed by the hydrolysis of a small molecule, GTP, associated with tubulin.
The current model of microtubule dynamics states that a microtubule elongates by incorporating GTP-bound tubulin at its end. Once incorporated, tubulin hydrolyzes GTP into GDP, releasing energy stored in the microtubule, making it unstable. The more tubulin molecules incorporated, the greater the accumulated energy until the microtubule becomes so unstable that it suddenly depolymerizes. The GDP-bound tubulin molecules released into the cytoplasm then exchange GDP for GTP and become competent again to reincorporate into a microtubule. This perpetual cycle of assembly and disassembly, coupled with GTP hydrolysis, gives microtubules their characteristic dynamic instability.
In the model just described, GDP-tubulin is considered inactive in microtubule assembly. However, the Team "Neurocytoskeleton Dynamics and Structure" managed by Isabelle Arnal and Annie Andrieux has shown that GDP-tubulin is actually capable of forming microtubules. These microtubules possess unique properties: they are remarkably stable and do not spontaneously disassemble.
These findings demonstrate that microtubules possess an intrinsic capacity for stability, independent of accessory proteins. Such stable microtubules could play a crucial role in differentiated cells such as neurons, where increased stability of the microtubule network is essential for proper function. Another example is axon regeneration after injury, which requires an appropriate balance between populations of dynamic and stable microtubules.
Reference :
Stable GDP-tubulin islands rescue dynamic microtubules.
Nassiba Bagdadi, Juliette Wu, Julie Delaroche, Laurence Serre, Christian Delphin, Manon De Andrade, Marion Carcel, Homaira Nawabi, Benoît Pinson, Claire Vérin, Yohann Couté, Sylvie Gory-Fauré, Annie Andrieux*, Virginie Stoppin-Mellet* and Isabelle Arnal*.
J Cell Biol (2024) 223 (8): e202307074. doi.org/10.1083/jcb.202307074
Date
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn