Directors : Isabelle ARNAL and Annie ANDRIEUX
Keywords
Research themes
The objective of the team “Neuro-cytoskeleton Dynamics and Structure” is to elucidate the mechanisms underlying cytoskeleton organisation in neuronal development and plasticity, and to understand how these mechanisms are deregulated in pathological conditions. More specifically, we aim at deciphering how MAPs -elaborate and control various MT arrangements -regulate actin behaviour and -contribute to the MT-actin crosstalk.
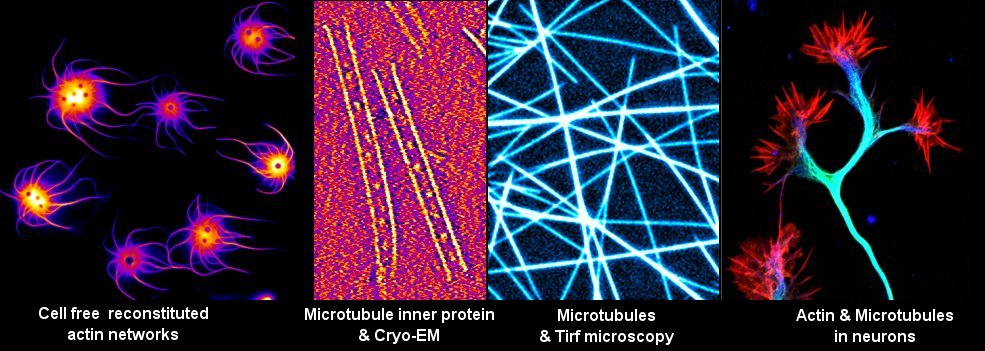
Microtubules, major cytoskeletal elements, are central to neuronal architecture and activity. Microtubules can be dynamic or stable and exist as single polymers or bundled networks. Microtubules cooperate with actin, another cytoskeletal component in neuron, this synergy being crucial for neuronal development and functions. By regulating microtubule stability and self-organisation, Microtubule-Associated Proteins (MAPs) are key microtubule regulators. Recent evidences including our work show that MAPs can also directly bind actin. Cytoskeleton alterations, including those resulting from MAPs dysfunctions, are linked to brain development disorders and neurodegenerative diseases.
Techniques used :
- Molecular biology and biochemistry: cloning, protein expression and purification, protein interactions
- Biomimetic assays: in vitro reconstitution of microtubule and actin behaviour
- Cell biology: primary cultures of neurons, subcellular localisation of proteins, cytoskeleton dynamics
- Microscopy: confocal and spinning disc microscopy, confocal airyscan microscopy, live imaging, Total-Internal Reflexion Fluorescence (TIRF) microscopy, single molecule TIRF imaging, expansion microscopy, electron cryo-microscopy and -tomography, 3D reconstructions (etomo, relion)
Partners : 



Thesis of the team
Publications
Serre L, Delaroche J, Vinit A, Schoehn G, Denarier E, Fourest-Lieuvin A, Arnal I. (2023) The mitotic role of adenomatous polyposis coli requires its bilateral interaction with tubulin and microtubules. J Cell Sci. Jan 15;136(2):jcs260152. doi: 10.1242/jcs.260152.
Fourest-Lieuvin A, Vinit A, Blot B, Perrot A, Denarier E, Saudou F, Arnal I. (2023) Controlled Tau Cleavage in Cells Reveals Abnormal Localizations of Tau Fragments. Neuroscience. May 10;518:162-177. doi: 10.1016/j.neuroscience.2022.08.016.
Peris L, Parato J, Qu X, Soleilhac JM, Lanté F, Kumar A, Pero ME, Martínez-Hernández J, Corrao C, Falivelli G, Payet F, Gory-Fauré S, Bosc C, Blanca Ramirez M, Sproul A, Brocard J, Di Cara B, Delagrange P, Buisson A, Goldberg Y, Moutin MJ, Bartolini F, Andrieux A. (2022) Tubulin tyrosination regulates synaptic function and is disrupted in Alzheimer's disease. Brain. Jul 29;145(7):2486-2506. doi: 10.1093/brain/awab436.
Boulan B, Ravanello C, Peyrel A, Bosc C, Delphin C, Appaix F, Denarier E, Kraut A, Jacquier-Sarlin M, Fournier A, Andrieux A, Gory-Fauré S, Deloulme JC. (2021) CRMP4-mediated fornix development involves Semaphorin-3E signaling pathway. Elife. Dec 3;10:e70361. doi: 10.7554/eLife.70361.
Cuveillier C, Boulan B, Ravanello C, Denarier E, Deloulme JC, Gory-Fauré S, Delphin C, Bosc C, Arnal I, Andrieux A. (2021) Beyond Neuronal Microtubule Stabilization: MAP6 and CRMPS, Two Converging Stories. Front Mol Neurosci. May 5;14:665693. doi: 10.3389/fnmol.2021.665693.
Cuveillier C, Delaroche J, Seggio M, Gory-Fauré S, Bosc C, Denarier E, Bacia M, Schoehn G, Mohrbach H, Kulik I, Andrieux, Arnal I, Delphin C (2020). MAP6 is an intraluminal protein that induces neuronal microtubules to coil. Science Adv 6:eaaz4344.
Serre L, Stoppin-Mellet V, Arnal I (2019) Adenomatous Polyposis Coli as a Scaffold for Microtubule End-Binding Proteins. J Mol Biol 431, 1993-2005.
Prezel E, Elie A, Delaroche J, Stoppin-Mellet V, Bosc C, Serre L, Fourest-Lieuvin A, Andrieux A, Vantard M, Arnal I (2018)
Tau can switch microtubule network organizations: from random networks to dynamic and stable bundles. Mol Biol Cell 29:154-165.
Peris L, Bisbal M, Martinez-Hernandez J, Saoudi Y, Jonckheere J, Rolland M, Sebastien M, Brocard J, Denarier E, Bosc C, Guerin C, Gory-Fauré S, Deloulme JC, Lanté F, Arnal I, Buisson A, Goldberg Y, Blanchoin L, Delphin C, Andrieux A (2018) A key function for microtubule-associated-protein 6 in activity-dependent stabilisation of actin filaments in dendritic spines. Nat Comm 9:3775.
Ramirez-Rios S, Denarier E, Prezel E, Vinit A, Stoppin-Mellet V, Devred F, Barbier P, Peyrot V, Sayas CL, Avila J, Peris L, Andrieux A, Serre L, Fourest-Lieuvin A, Arnal I (2016) Tau antagonizes end-binding protein tracking at microtubule ends through a phosphorylation-dependent mechanism. Mol Biol Cell 27:2924-2934.
Elie A, Prezel E, Guérin C, Denarier E, Ramirez-Rios S, Serre L, Andrieux A, Fourest-Lieuvin A, Blanchoin L, Arnal I (2015) Tau co-organizes dynamic microtubule and actin networks. Sci Rep 5:9964.
Members
- Annie ANDRIEUX
- Adrien ANTKOWIAK
- Isabelle ARNAL
- Christophe BOSC
- Nicolas CHAUMONTEL
- Manon DE ANDRADE
- Julie DELAROCHE
- Christian DELPHIN
- Eric DENARIER
- Anne FOUREST-LIEUVIN
- Sylvie GORY-FAURE
- Virginie LAGIER
- Kelig LE CORRE
- Laurence SERRE
- Virginie STOPPIN-MELLET
- Juliette WU