- Share
- Share on Facebook
- Share on X
- Share on LinkedIn
Easy-to-use microscopes
For a simple control, or an image acquisition...
NIKON Eclipse TS100

A control microscope to check the samples before using a more advanced system.
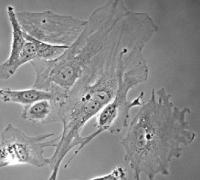
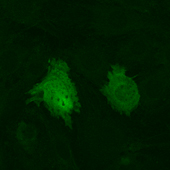
Video microscopes
Wide field microscopes are meant to be used for monitoring the development of fluorescent and non-fluorescent cells over short or long periods of time. In order to minimize the degradation of the observed samples during the acquisition, a balance must be found by favoring short exposure times and fast illumination changes. Some wide field microscopes are fitted with a temperature and/or CO2 control system which ensures that the samples survive for extended periods. Motorized stages allow the memorization of several areas and to acquire their images one after another during the same experiment. These tools are easy to use and meet most needs. Their weakness lies in the fact that the whole field of view is illuminated. This causes a lack of resolution, especially for thick samples, which may necessitate the use of laser scanning confocal microscopes or spinning disk microscopes.
Services
- XYZT observation
- Acquisition on short or long periods of time
- Multi-marking images
- Thermoregulation & CO2 control for maintaining samples
- Multi-positioning for monitoring several fields during the acquisition
- Live or fixed samples
- Multiple sample holder options (Petri dishes, slides, multiwell dishes,…)
Access mode
- Assistance
- Autonomy (after training)
- Provision of services on request
Equipements
- LEICA DMI6000 / ROPER
Inverted, thermoregulation, multipositioning, scan slides, FRAP and photoactivation, calcium imaging

- ZEISS AxioObserver
Inverted, thermoregulation & CO2 control, multipositioning, acquisitions and Time Lapse

- ZEISS Axiovert 200M
Inverted microscope for immunolabelling visualization

Microscope confocal Spinning Disk
The ideal combination of high resolution and low phototoxicity. The "spinning-disk" combines the rapidity of a wide field microscope and the high resolution of a confocal. This technique uses lasers to illuminate the samples and ultra-sensitive cameras to detect the fluorescent signal. Contrary to the laser scanning confocal microscope, the spinning disk simultaneously illuminates the whole area of observation, reducing the acquisition time and phototoxicity, thus enabling access to 4D imaging (3D + time).
Services
- XYZT fluorescence and/or white lightacquisition
- Multi-labelling
- Acquisitions on short or long periods of time
- Multi-marking images
- Thermoregulation & CO2 control for maintaining samples
- Fast in depth motion and precise repositioning (Piezo table)
- Multi-positioning for monitoring several fields during the acquisition
- Live or fixed samples
- Multiple sample holder options (Petri dishes, slides,…)
- High resolution acquisitions
Access mode
- Support
- Autonomy (after training)
- Service provision on request
Equipments
- SPINNING DISK ZEISS Axio Observer Z1 / ROPER
Combination of high resolution and low phototoxicity

- LIVE SR Module
Confocal / two photon Microscopes
The PIC GIN optical microscopy platform has several confocal microscopes. They deliver fine optical sections of a tissue or a cell by detecting only the fluorescent signal created by the focal plane while getting rid of the upper and lower planes' stray fluorescence, in contrast to wide field microscopy. They provide a very high axial resolution but the samples will be damaged by the phototoxicity caused by laser sources, thus diminishing the ability to observe the sample over time. Spinning disk-type systems can offset the drawbacks of confocal microscopy as well as its relatively slow-speed acquisition (point-by-point laser scanning) when studying life science.
Laser scanning microscopes can be divided into two categories: single-photon confocal microscopes and two-photon confocal microscopes.
Two-photon microscopy uses an IR pulsed femtosecond laser which penetrates deeper into the sample (1mm) and minimizes the chemifluorescent photobleaching and photodamaging of the sample. Two-photon microscopy excitation is created only by the focal point, which gives optical sections of thick samples (without any pinhole) and this makes a 3D reconstruction possible. Two-photon microscopy combined with photoactivation (optogenetics, glutamate uncaging…) and electrophysiology enables in vivo functional imaging (calcium imaging…) of individual cells (neurons, astrocytes) in anesthetized rodent brains.
Applications
- XYZT observation (multipositioning) and overtime
- Multi-marking images
- UV lasers for nuclear marking (DAPI, Hoechst)
- Thermoregulation & CO2 control for maintaining samples
- Live or fixed samples
- Spectral analysis
- FRAP, FRET, 2D STED
- Module AIRYSCAN (High Resolution)
Access mode
- Support
- Autonomy (after training)
- Provision of services on request
Equipments
- ZEISS LSM710/AIRYSCAN
Inverted, analysis, spectral, thermoregulation, FRAP, FRET, high resolution

- LEICA TCS SPE
Inverted, no UV laser, no regulation

- ZEISS 7MP
Upright microscope, 3 NDD (epifluorescence), long-travel motorized stage, gas anesthesia, IR laser 680-1080m

- STEDYCON ABBERIOR
Inverted microscope, 4 channels for confocal microscopy and 2 channels for 2D STED ( excitation lasers 561 and 640nm, depletion laser 775nm)
- LAVISION BIOTEC TriMScope

|
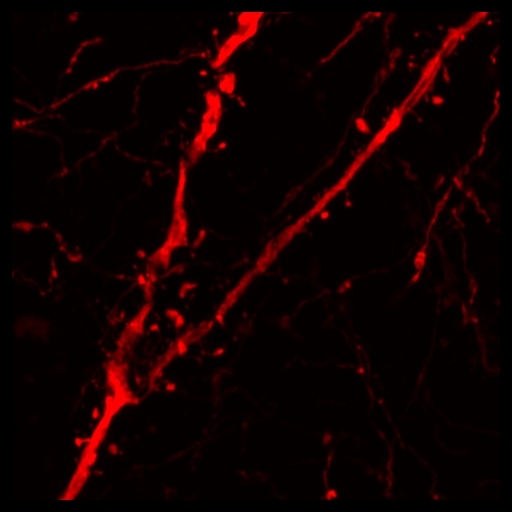
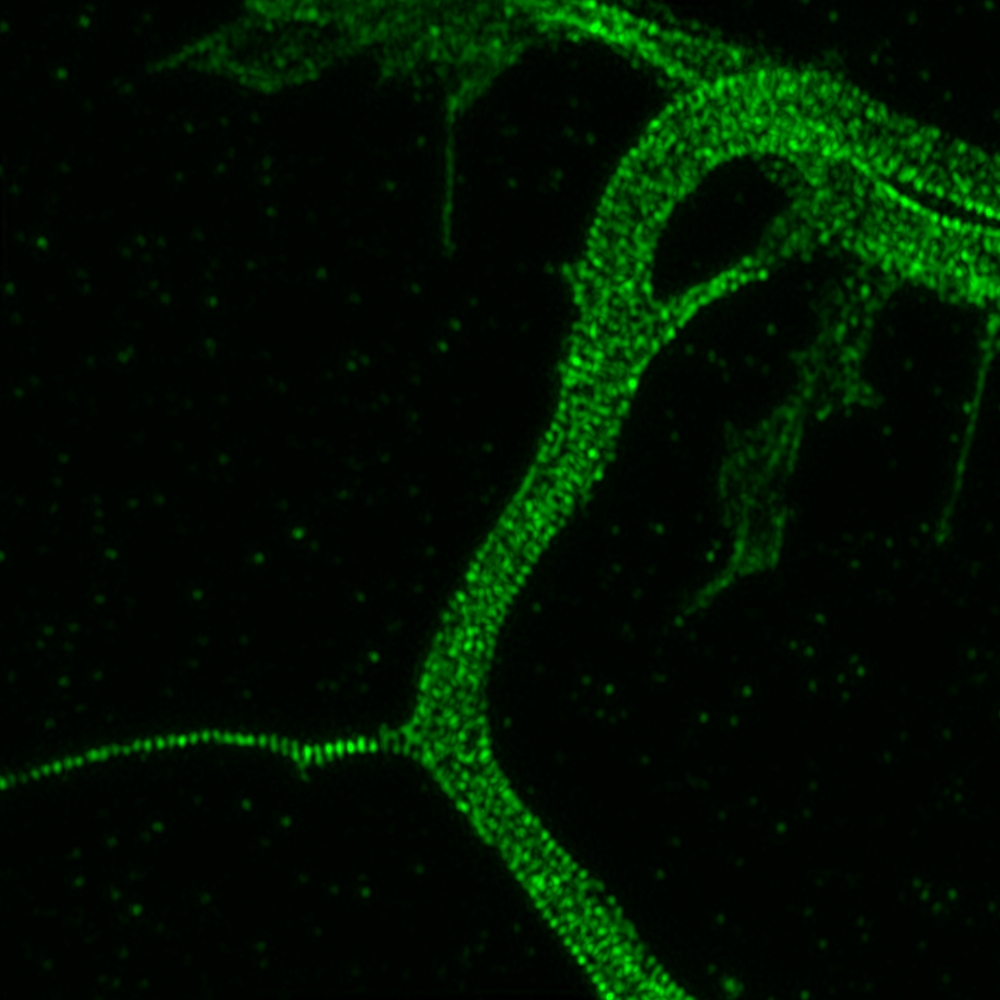
Confocal microscopy Images
|
|
|---|---|
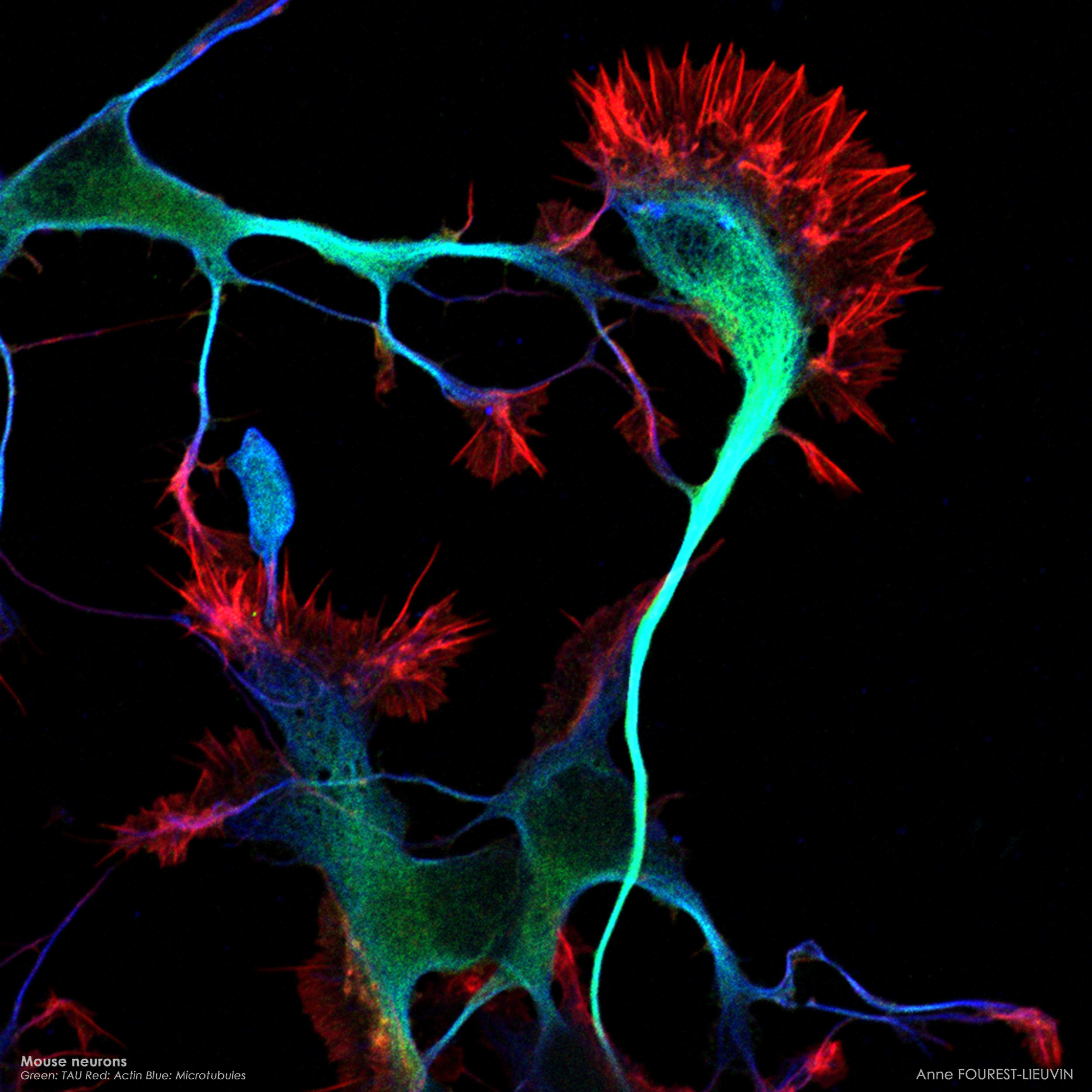
(Anne Fourest lieuvin) |
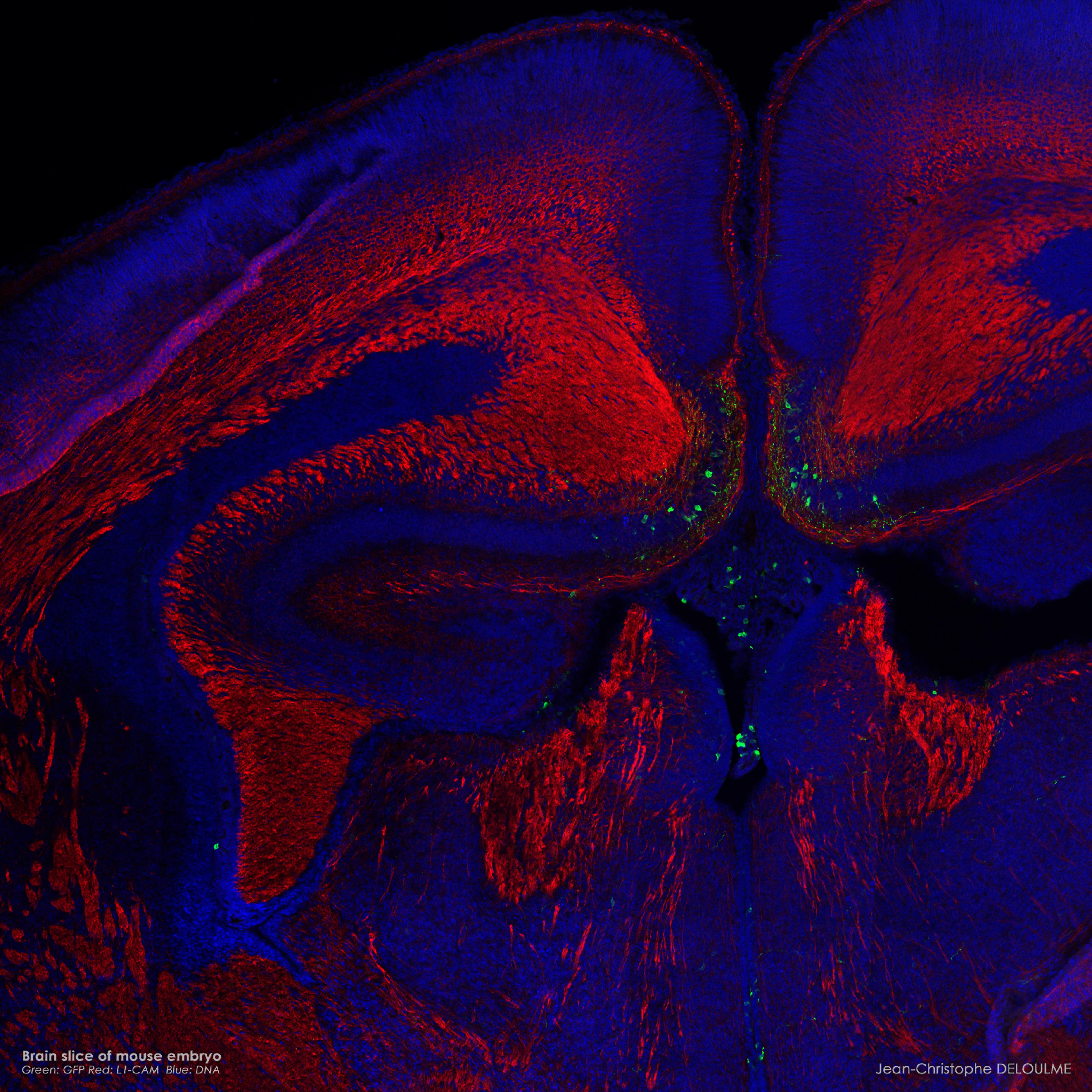
(Jean-Christophe Deloulme) |
|
Biphoton microscopy Images
|
|
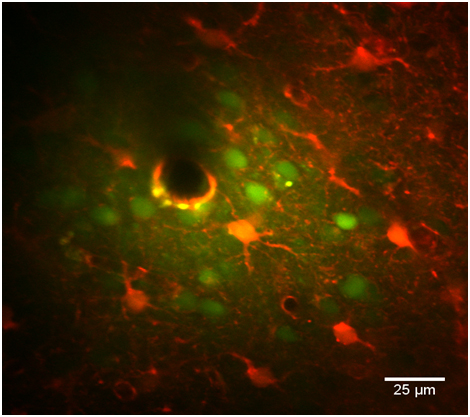
(Florence Appaix) |
(Florence Appaix) |
TIRF Microscope
TIRF (Total Internal Reflection Fluorescence) microscopy generates images of an optical section from 20 to 200nm thick. With TIRF, only the part of the sample's surface closest to the slide is observed. This microscope, with its high axial resolution, is a major instrument to observe membranes or to study the cytoskeleton.
Applications
- Study of membrane receptors
- Study of exocytosis/endocytosis
- Study of cell/substrate adhesion
- Study of the cytoskeleton underneath the membrane
- Study of single molecules (smTIRF)
Access mode
- Support
- Autonomy (after training)
- Provision of services on request
Equipment
- NIKON / ROPER
Inverted, thermoregulation
High Resolution
AIRYSCAN
New Confocal Technology to improve sensitivity and resolution
With the new AIRYSCAN detector installed on the LSM 710 ZEISS confocal, users can achieve 1.7 × higher resolution in all spatial dimensions. Improved sensitivity gives better image quality. The entire imaging process can be performed with sample preparation protocols and standard immunolabelling.
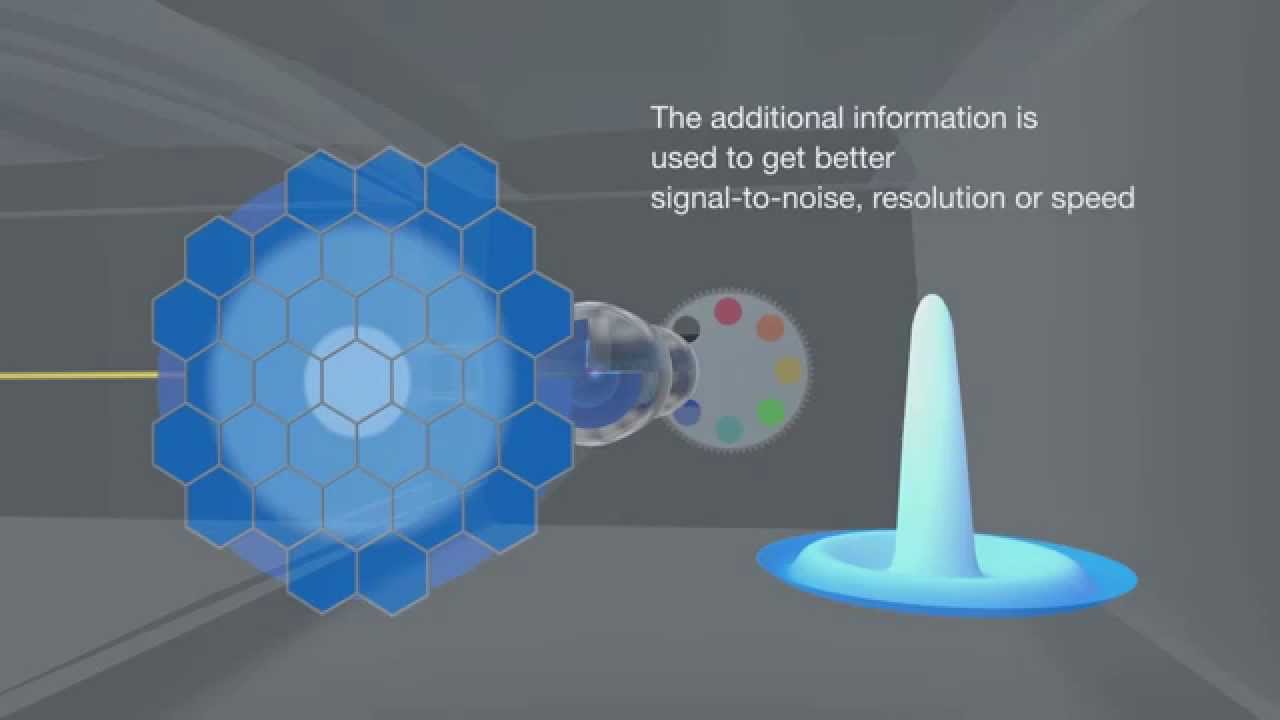
LIVE SR
Live-SR is based on optically demodulated structured illumination technique with online processing. Combined with spinning confocal, it enables High Resolution to be achieved at high making it the ideal solution for live high resolution cell imaging.

Slide scanner
Slide scanner equiped with a 100 slide magazine.
It allows the scanning of samples in transmitted light, polarization and fluorescence and the creation of virtual slides.
Applications
- Fixed samples only
- Immunolabelling, immunohistochemistry (TMA, brain mouse cut, rat ...), polarization, transmitted light
Access mode
- Support
- Autonomy (after training)
- Service provision on request
Equipment
- ZEISS : Axioscan Z1

Slide Scanner
Lightsheet Microscope
Light sheet installation in February 2019
This system will allow the acquisition of macroscopic images in fluorescence and in 3D.
Applications
- Clearing samples
Access mode
- Support
- Autonomy (after training)
- Service provision on request
Equipment
- LAVISION BIOTEC : Ultramacroscope II

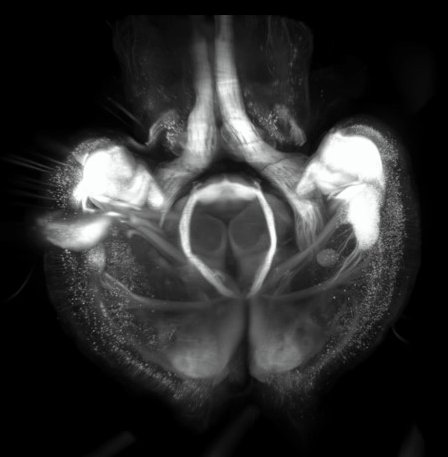
Super resolution microscopy
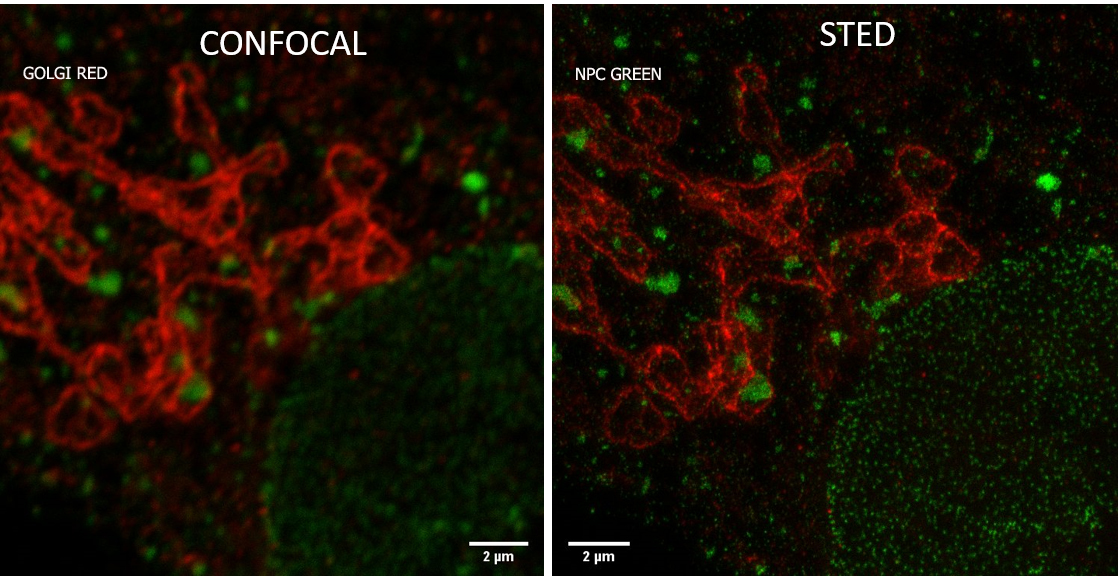
- STEDYCON 2D (installation Decembrer 2021)
With the new STEDYCON module (Abberior) installed on a conventional epifluorescence microscope (Zeiss), users will have access to a new confocal microscope and 2D STED superresolution microscopy. Super resolution acquisition via the STEDYCON module allow ~40nm lateral resolution depending on the fluorochrome and the objective.
Applications
- XYZ confocal acquisitions in fluorescence
- Multilabelling until 4 fluorochromes
- Speed depth movement and precise repositioning (Piezo on objective)
- Fixed samples only
- Samples support (Petri dish, slide, …)
- Super resolution 2D STED acquisition with 2 wavelengh
Access mode
- Support
- Autonomy (After training)
- Prestations on request
Equipment
- STEDYCON: Abberior


Sterology microscopy
Installation in June 2023
Stereology is method for quantitative histology.
It is used to accurately quantify the number of cells, the length of fibers, and the area and volume of biological structures or regions.
Applications
- XYZ acquisitions and quantitative analysis
Access mode
- Support
- Autonomy (After training)
- Prestations on request
Equipment : MBF Biosciences
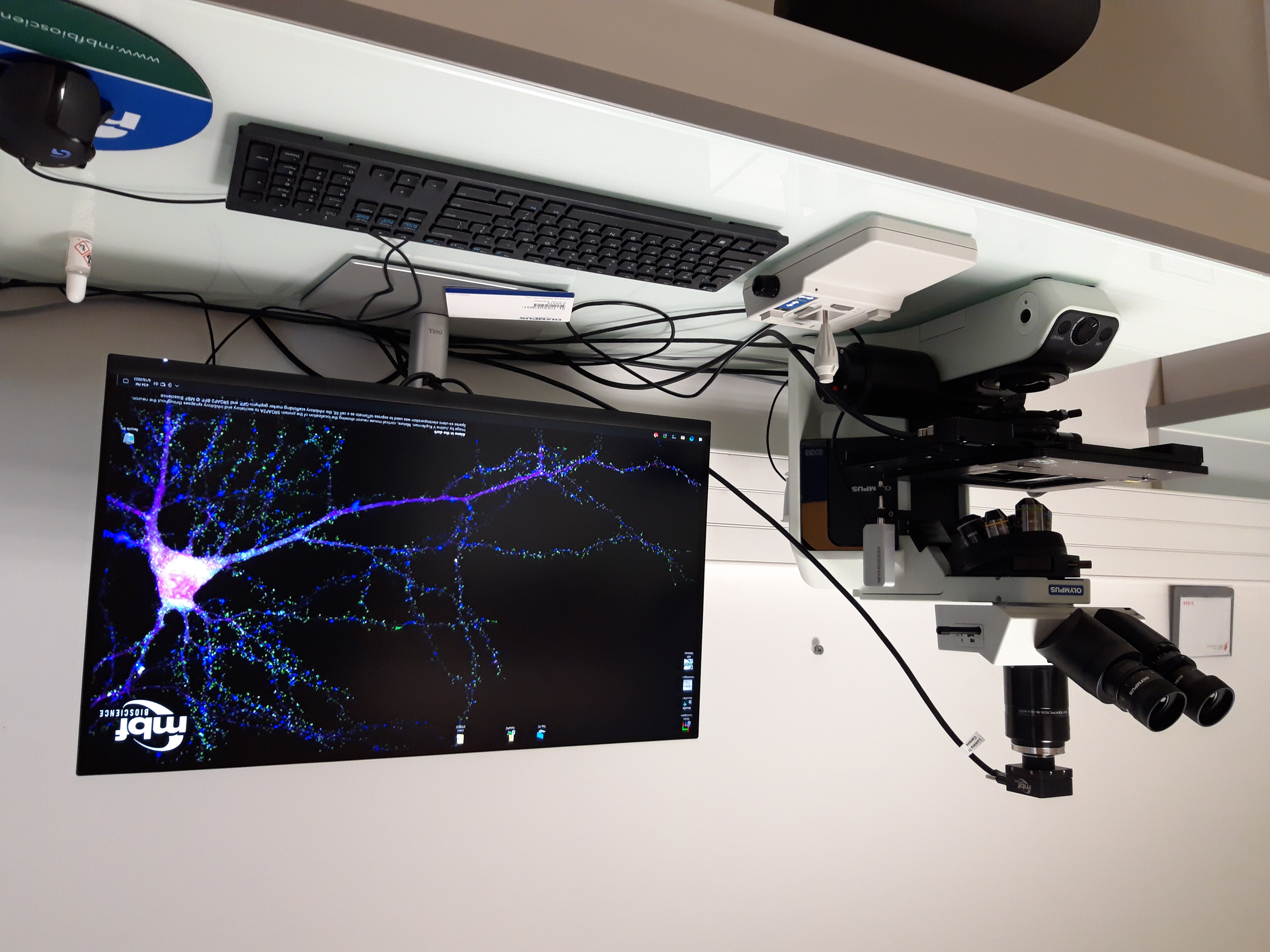
- Microscope : BX 53 Olympus
•Objectifs : x2, x10, x20, x40, x60, x100
•Camera : color
•Software : StereoInvestigator
Analysis Room
A computer room is available to users of the platform for data processing.
Access mode
- Support
- Autonomy (after training)
- Service provision on request
Equipment
- IMARIS software, Zen Blue, MetaMorph, Fiji are installed on PC
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn