Director : Alain BUISSON
Keywords
Themes of research
The team "Neuropathologies and synaptic dysfunctions" develops a project to characterize the molecular mechanisms underlying early Alzheimer's disease by focusing on the synapse which represents the first target of the disease.
The team recently expanded into a group that is interested in microtubules in the synapse and a particular modification of neuronal tubulin, detyrosination.
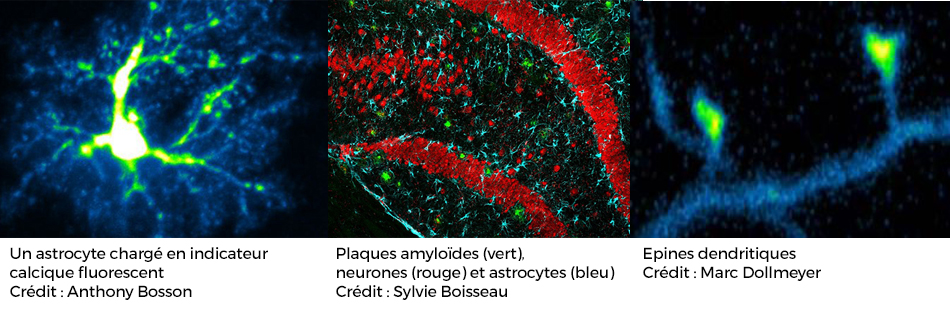
Alzheimer disease is characterized at the cellular level by the accumulation of proteins outside (beta-amyloid) and inside neurons (fibrillar proteins associated with hyperphosphorylation of tau protein). Many studies have shown that the decrease in the number of dendritic spines (contact area between synaptic neurons) appears early in the disease development and is strongly correlated with the cognitive deficits observed in patients (including memory loss). The team has developed tools to study the early effects of these accumulations on the function and the structure of the synapse.
To characterize the molecular mechanisms leading to the loss of synaptic plasticity in Alzheimer's disease, the team "Neuropathologies and synaptic dysfunctions" focuses on the effects of ? amyloid peptide accumulation on synapse function and develops the following research projects:
- Functional plasticity of glutamatergic synapses: to characterize intracellular and extracellular targets of ? amyloid peptide and the consequences of its accumulation on pathological dysfunctions of glutamatergic neurotransmission, including the functioning of NMDA receptors.
- Structural plasticity of glutamatergic synapses: to determine the effects of ? amyloid peptide accumulation on the actin cytoskeleton. Actin is the main synaptic component controlling the shape, the organization and the function of dendritic spines.
- Involvement of astrocytes in these synaptic dysfunctions: to study the modifications of the communication between astrocytes and neurons and how the structural and functional plasticity of astrocytes are linked to the synaptic loss.
Techniques used :
-
Physiology: electrophysiology and calcium imaging in brain slices and cell cultures
-
Biochemistry and Cell Biology: primary cultures of neurons and astrocytes, recombinant protein expression, purification of synaptosomes (pre- and postsynaptic fractions), purification of gliosomes, protein interaction studies
-
Optical Microscopy: Confocal microscopy, FRAP
-
APP/PS1-21 transgenic mice (Alzheimer disease animal model)
Partners : 
Thesis of the team (in french)
Publications
Major publications
Paumier A, Boisseau S, Jacquier-Sarlin M, Pernet-Gallay K, Buisson A, Albrieux M (2021 July). Astrocyte-neuron interplay is critical for Alzheimer's disease pathogenesis and is rescued by TRPA1 channel blockade Brain PMID: 34302466; DOI: 10.1093/brain/awab281
Bosson A, Paumier A, Boisseau S, Jacquier-Sarlin M, Buisson A and Albrieux M (2017). TRPA1 channels promote astrocytic Ca2+ hyperactivity and synaptic dysfunction mediated by oligomeric forms of amyloid-beta peptide. Mol Neurodegener., 12 (53): 1-19.
Frandemiche ML, De Seranno S, Rush T, Borel E., Elie A., Arnal I., Lanté F. and Buisson A. (2014). Activity dependent tau protein translocation to excitatory synapse is disrupted by exposure to Amyloid ? oligomers. J. Neurosci., 34:6084-6097
Lanté F, Toledo-Salas JC, Ondrejcak T, Rowan MJ, Ulrich D. (2011). Removal of synaptic Ca²+-permeable AMPA receptors during sleep. J Neurosci. 31:3953-61.
El Gaamouch F, Buisson A, Moustié O, Lemieux M, Labrecque S, Bontempi B, De Koninck P, Nicole O. (2012). Interaction between ?CaMKII and GluN2B controls ERK-dependent plasticity. J Neurosci. 1;32(31):10767-79.
Bordji K, Becerril-Ortega J, Nicole O, Buisson A (2010). Activation of Extrasynaptic, But Not Synaptic, NMDA Receptors Modifies Amyloid Precursor Protein Expression Pattern and Increases Amyloid-? Production. J. Neurosci. 30:15927–15942
Bosson A, Boisseau S, Buisson A, Savasta M, Albrieux M (2015). Disruption of dopaminergic transmission remodels tripartite synapse morphology and astrocytic calcium activity within substantia nigra pars reticulata. Glia. 63 (4): 673–68
Members
- Mireille ALBRIEUX
- Martina ALEMAN
- Sylvain ANDRIEU
- Guillaume AUDIC
- Sylvie BOISSEAU
- Eve BOREL-MENEROUD
- Alain BUISSON
- Nour CHAKROUN LAMIRI
- Yves GOLDBERG
- Samar ISMAIL
- Muriel JACQUIER-SARLIN
- Fabien LANTE
- Maximiliano MELANO
- Marie-Jo MOUTIN
- Leticia PERIS
- Apolline PIERRE
- Sacnicte RAMIREZ RIOS
- Quentin RODRIGUEZ
- Maxime SEIGNOBOS
- Aditi SHARMA
- Jean Marc SOLEILHAC
- Marion TUDURY