Manager : Michael DECRESSAC
Keywords
Themes of research
Age-related neurodegenerative diseases represent a major socio-economical burden for our society and a fundamental challenge for the scientific community. Ameliorating brain healthspan (i.e our lifetime spend with good cerebral function) is an unmet need that can only be achieved by improving our knowledge on neuronal physiology.
The goal of the team “Brain aging and repair” is to decipher the molecular signals governing neuronal senescence. Their identification would allow the elucidation of the pathogenic mechanisms underlying age-related neurodegenerative diseases such as Parkinson’s disease. Addressing this critical issue would also accelerate the discovery of pertinent and effective therapies for patients.
We are particularly interested in understanding how the disease-causing protein alpha-synuclein becomes toxic and what is the sequence of the pathogenic events leading to the stochastic acceleration of nigral dopamine neurons loss in Parkinson’s disease. We study the extra-cellular signals and the intra-cellular mechanisms regulating its expression level and the consequences at molecular and cellular levels when its homeostasis is perturbed. Our group is pursuing the following lines of research:
To address these questions, the “Brain aging and repair” team develops the following research themes:
- Pathogenic mechanisms : we try to elucidate the signals originating from the brain or from peripheral organs that regulate cerebral aging and prime the brain towards the development of pathologies.
- Disease modeling : we design alternative and relevant strategies (e.g gene transfer) to mimic Parkinson’s disease in cells and in in vivo.
- Molecular therapy : we use unbiased approaches to identify clinically approved drugs for repurposing in Parkinson’s disease. By screening libraries against the druggable genome we also identify target genes to test in complementary models of pathological brain aging.
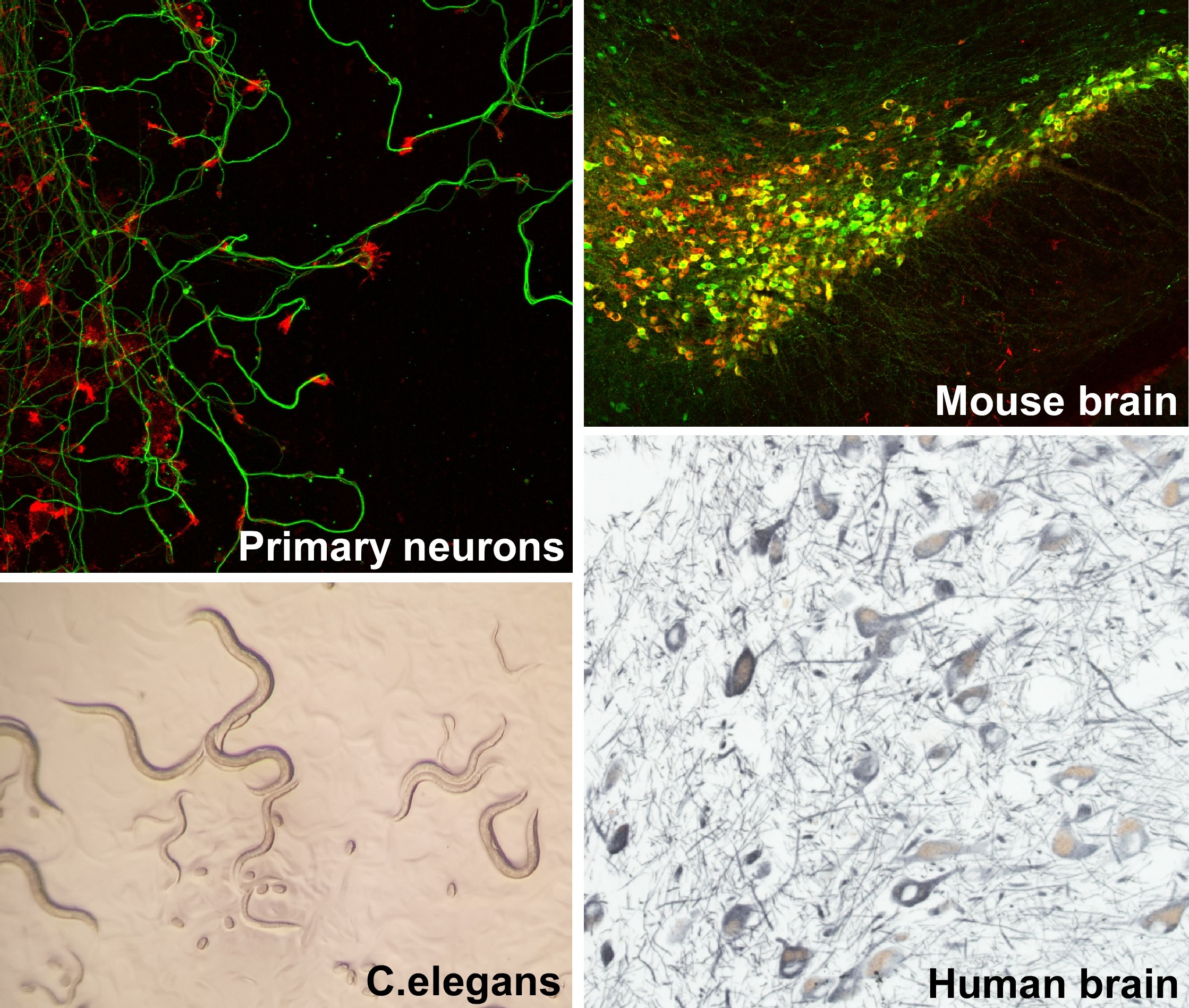
Legend: Translational approach to study pathological brain aging. Our research models span from primary neurons (top left: cortical neurons stained for tuj1 and phalloidin) to powerful genetic models such as C.elegans (bottom left) and transgenic mice (top right: substantia nigra stained for TH in red and GFP in green). We complement the clinical relevance of our findings by the examination of post-mortem tissues from patients ""(bottom right: nigral dopamine visualized with neuromelanin in brown and TH staining in grey)."
Techniques used
- Microscopy : immunohistochemistry, immunofluorescence confocal and live imaging, electron and super-resolution microscopy
- Biochemistry, Molecular and cell biology : cloning, RT-qPCR, transfection, viral transduction, ELISA, Western Blot
- Research models : cell lines, primary neuronal and glial cultures, c.elegans, mice, rats, humans
- In vivo : viral vectors delivery, stereotaxic surgery, behavioral phenotyping
Partners : 
Thesis of the team
Publications
Nurr1 in Parkinson’s disease: from pathogenesis to therapeutic potential. Decressac M., Volakakis N., Bjorklund A., Perlmann T. (2013) Nat. Rev. Neurol. 9(11):629-36
Cyclosporin promotes neurorestoration and cell replacement therapy in pre-clinical models of Parkinson’s disease. Tamburrino A., Churchill M.J., Wan O.W., Colino-Sanguino Y., Ippolito R., Bergstrand S., Wolf D.A., Herz N.J., Sconce M.D., Bjorklund A., Meschul C.K., Decressac M. (2015) Acta Neuropathol. Commun. 3:84.
TFEB-mediated autophagy rescues midbrain dopamine neurons from a-synuclein toxicity. Decressac M., Mattsson B., Weikop P., Lundblad M., Jakobsson J., Bjorklund A. (2013). PNAS, 110(19):E1817-26.
a-synuclein –induced down-regulation of Nurr1 disrupts GDNF signaling in nigral dopamine neurons. Decressac M., Kadkhodaei B., Mattsson B., Laguna A., Perlmann T., Bjorklund A. (2012) Sci. Transl. Med. 4(163):163ra156.
Members
- Mathilde DECRESSAC
- Michael DECRESSAC
- MME Marjorie HERNANDEZ