- Share
- Share on Facebook
- Share on X
- Share on LinkedIn
Communiqué / Team J.Bastin, Parkinson
On May 21, 2025
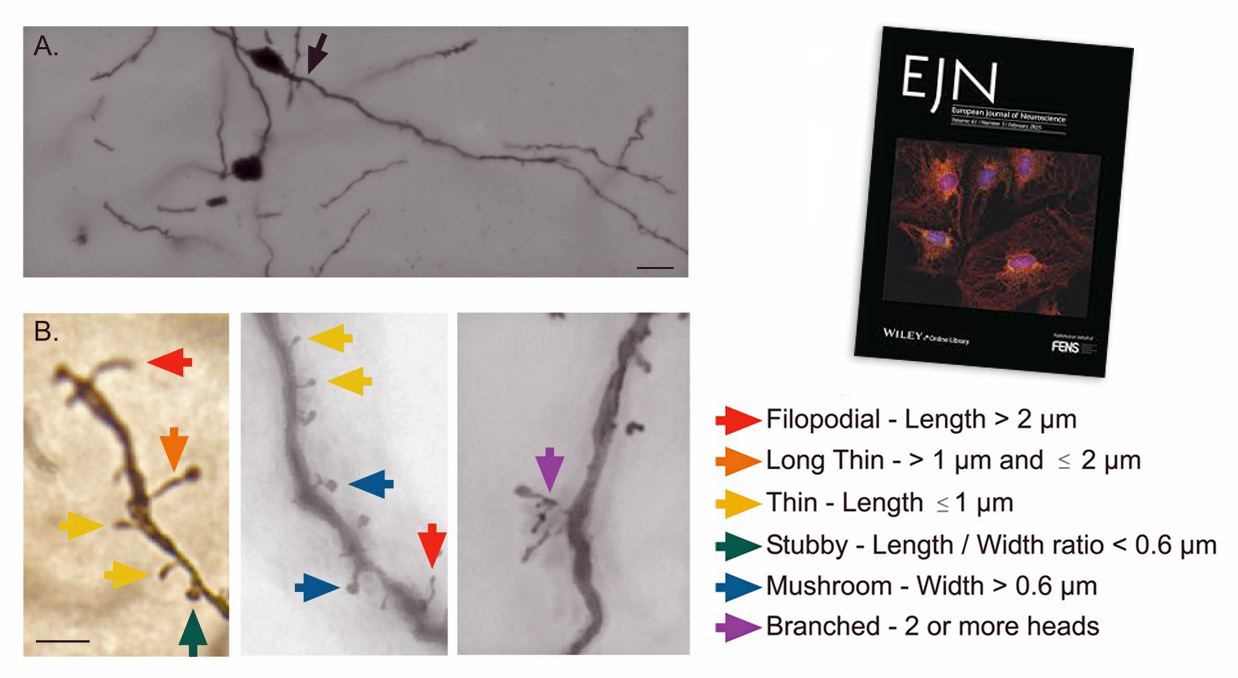
The impact of Parkinson's disease on the brainstem has so far been little studied. However, GIN researchers have just shown that some of its structures are able to reorganize themselves in Parkinsonian rats, providing evidence of specific neuroplasticity.
Parkinson's disease is a neurodegenerative disorder characterized by the progressive loss of dopaminergic neurons. Following this loss, GABAergic neurons in the substantia nigra pars reticulata, an output structure of the basal ganglia, become hyperactive.
“Our hypothesis was therefore that the increased release of GABA by these neurons should induce neuroadaptations in the brainstem structures receiving them, notably the colliculus (superior and medial) and the periaqueductal gray”, explains Véronique Coizet, a researcher at the GIN in the ‘Brain, Behavior and Neuromodulation’ team and author of the publication. “The aim of this study was to assess cellular and molecular plasticity in these structures, in rats with Parkinson's disease that had undergone partial or total dopaminergic lesioning.”
At the anatomical level, the neuroscientists used the Golgi-Cox method to measure the density and morphology of dendritic spines. At the molecular level, they used the Western blot technique to analyze the expression of type A GABA receptors.
Their results show an increase in the density of “Thin” and “stubby” dendritic spines in the colliculus and periaqueductal gray, but only in the case of total dopaminergic lesions.
However, the increase in GABA type A receptor expression was only observed in the lateral colliculus and in the group with total dopaminergic lesions.
“Finally, we observed differences in plasticity in these brainstem structures, suggesting the existence of compensatory mechanisms in Parkinson's disease, which may delay onset and contribute to motor and non-motor symptoms in the patient” concludes Véronique Coizet.
Further investigation should be performed to fully understand the functional impact of the plasticity revealed in this work.
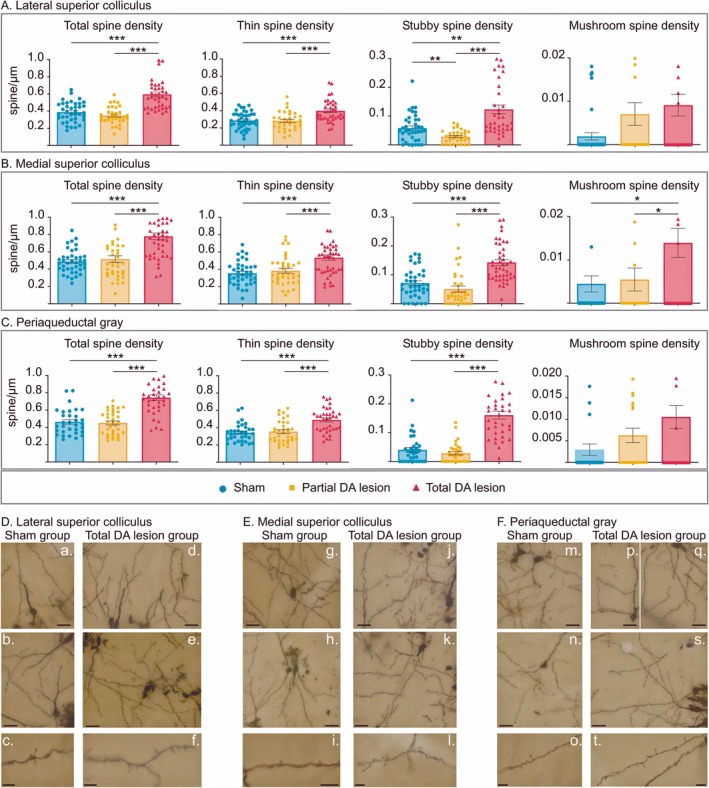
Top panel: histograms (mean ± SEM) of the total spine, thin spine, stubby spine, and mushroom spine density measured in tissue labeled with Golgi–Cox staining in the sham (blue), partial (orange), and total (red) DA lesion groups within the lateral superior colliculus (A), the medial superior colliculus (B), and the periaqueductal gray (C). Bottom panel: photomicrograph montages (merge of different Z axes) of dendritic spines labeled with Golgi–Cox staining in the lateral superior colliculus (D), the medial superior colliculus (E), and the periaqueductal gray (F) in the sham (a–c, g–i, m–o) and total DA lesion groups (d–f, j–l, p, q, s, t). Scale bar = 20 μm (top and middle pictures). Scale bar = 5 μm (bottom pictures). *p < 0.05, **p < 0.01, and ***p < 0.001.
Référence :
Brainstem Neuroadaptations in Rodent Models of Parkinson's Disease
Racha Al Tannir , Arnaud Pautrat , Remi Soutrenon , Paul G Overton , Veronique Coizet
Eur J Neurosci. 2025 Mar 31;61(7):e70068. doi: 10.1111/ejn.70068
Date
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn